Abstract
Aims: Patients with the cutaneous T-cell lymphoma subtypes mycosis fungoides (MF) and Sézary syndrome (SS) often require multiple lines and types of systemic therapy. The phase 3 MAVORIC study (NCT01728805) showed that mogamulizumab (MOGA), a monoclonal antibody directed against C-C chemokine receptor 4 (CCR4), is superior to vorinostat (VORI) in median progression-free survival (PFS; 7.7 vs 3.1 months, P<0.0001) and confirmed overall response rate (ORR [complete response plus partial response]; 28% vs 4.8%, P<0.0001) in patients with MF/SS with a median of three prior systemic therapies (range: 1-18). Preclinical studies have suggested that histone deacetylase inhibitors (HDACi) may downregulate CCR4 expression in neoplastic T-cells. Further, romidepsin (an HDACi) has also been shown to suppress NK cell function, which could negatively influence the antibody activity of MOGA. As a result, this post hoc analysis of the MAVORIC study examined the effect of prior systemic therapies, including romidepsin, on response to MOGA.
Methods: Patients with MF/SS who had failed ≥1 systemic therapy were randomized to MOGA 1.0 mg/kg intravenously or VORI 400 mg orally daily until disease progression or unacceptable toxicity. Confirmed ORR was based on a global composite response score in each of four disease compartments (skin, blood, lymph nodes, and viscera), achieved at two consecutive visits. PFS, ORR, and the interaction with time from treatment were assessed based on immune activity of last prior regimens, and analyzed using Cox proportional hazards and logistic regression models, respectively.
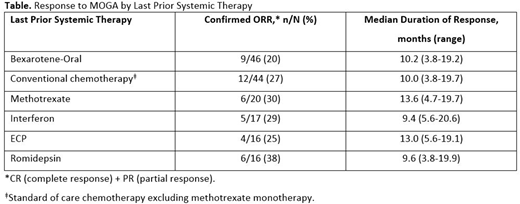
Results: In total, 372 patients (median age: 64 years) were randomized (MOGA, n=186; VORI, n=186). Baseline characteristics, including number and type of prior systemic therapies, were similar between cohorts. The most common last prior systemic therapies in patients randomized to MOGA were oral bexarotene (n=46; 25%), chemotherapy (n=44; 24%), methotrexate (n=20; 11%), interferon alpha (n=17; 9%), extracorporeal photophoresis (ECP; n=16; 9%), and romidepsin (n=16; 9%). Confirmed ORRs in MOGA-treated patients after 1, 3, or ≥6 prior therapies were 25%, 35%, and 30%, respectively. Patients who crossed over to MOGA from VORI, due to progression or intolerance, had an ORR of 30%. ORR and duration of response to MOGA did not vary by last prior systemic therapy (Table). Logistic regression analyses demonstrated that neither the impact of immune activity of the last prior therapy (immune stimulatory or immunosuppressive regimens) nor the time from prior treatment had an effect on PFS or ORR observed in response to MOGA (P>0.05)
Conclusions: This post hoc analysis of the MAVORIC study shows no difference in MOGA response by the number of prior systemic therapies, type, or immune activity of last prior therapy.
Zinzani:MSD: Honoraria, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Speakers Bureau. Horwitz:Spectrum: Research Funding; Innate Pharma: Consultancy; Forty Seven: Consultancy, Research Funding; Trillium: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Kyowa-Hakka-Kirin: Consultancy, Research Funding; Millennium/Takeda: Consultancy, Research Funding; Portola: Consultancy; Celgene: Consultancy, Research Funding; Mundipharma: Consultancy; Aileron Therapeutics: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Corvus: Consultancy. Kim:Medivir: Membership on an entity's Board of Directors or advisory committees; Portola: Research Funding; miRagen: Research Funding; Neumedicine: Consultancy, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Kyowa-Kirin-Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tetralogic: Research Funding; Galderma: Research Funding; Innate Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Horizon Pharma: Consultancy, Research Funding; Soligenix: Research Funding; Merck: Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Forty Seven Inc: Research Funding. Moskowitz:ADC Therapeutics: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Bristol Myers-Squibb: Consultancy, Research Funding; Merck: Research Funding; Incyte: Research Funding; Takeda: Honoraria. Porcu:Innate Pharma: Consultancy. Scarisbrick:National Health System, UK: Employment; Kyowa: Consultancy; Takeda: Consultancy; Helsinn: Consultancy; Actellion: Consultancy; Mallinkcrodt: Consultancy; 4SC: Consultancy; Innate Pharma: Consultancy. Leoni:Kyowa Kirin: Employment. Dwyer:Kyowa Kirin: Employment. Sun:Kyowa Kirin: Employment. Nikonova:Kyowa Kirin: Employment. Bagot:Takeda: Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees; Innate Pharma: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Actelion: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal